
The time taken for this to happen provides a surprisingly accurate measure of the relative activity of the enzyme under different conditions. The bubbles of oxygen generated by the decomposition of the peroxide stick to the filter paper circle and carry it to the liquid surface. Soak one of these circles in the enzyme extract, drain, then use a glass rod to poke it to the bottom of a test tube containing the hydrogen peroxide solution. Next, use a file paper hole punch to stamp out circles from a filter paper. There is also another, more novel, approach to monitoring the enzyme-catalysed reaction which you might like to try. You can do this by connecting a glass gas syringe to a Buchner flask or Hirsch tube. As an alternative you might prefer to measure the volume of oxygen produced during the decomposition of the hydrogen peroxide. The volume of thiosulphate solution required for the titration provides a measure of the amount of hydrogen peroxide in the reaction mixture at the different times. The iodine produced can be titrated with a solution of thiosulphate using starch indicator: You can do this by taking samples of the reaction mixture at different times and running them into an acidified solution of potassium iodide. You might decide to follow the fall in concentration of the hydrogen peroxide. There are several ways in which you can monitor the decomposition reaction. You might like to investigate how the effectiveness of this catalyst changes under different conditions and compare it with the other catalysts.
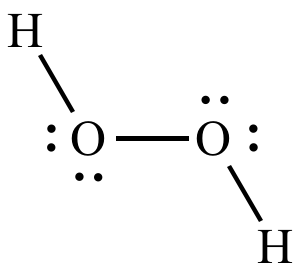
In this method the l8 December, 2008 clean it and then the remaining solution is decomposed with the help of a platinum-coated catalyst. The catalytic decomposition of hydrogen peroxide will be very familiar to some students, as it is an integral part of one system for cleaning contact lenses. Experiments of this kind could lead you towards a possible reaction mechanism and towards the calculation of the activation energy involved. You could set out to look at how factors such as concentration of the peroxide, amount of the catalyst and temperature affect the rate of the decomposition. Using an inorganic catalystĪs an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese(IV) oxide or lead(lV) oxide. Such experiments may at first sight seem quite straightforward, but as you try to interpret your results you will be led into quite complex, but very interesting, aspects of chemical kinetics and reaction mechanisms. This might lead you on to a more thorough investigation of other factors that affect the activity of the catalase, including, perhaps, temperature, pH, concentrations of substrate and enzyme and the presence of enzyme inhibitors. The rate of decomposition is increased by the intra-cellular enzyme catalase.Īs a fairly simple project you could compare the effectiveness of the enzyme from different sources such as potato, celery and liver. In many living organisms hydrogen peroxide is a product of metabolism that must be broken down, since in appreciable concentrations it is toxic. This is because of the variety of catalysts that will increase the rate of decomposition and the methods that can be used to monitor the reaction:ĢH 2O 2 → 2H 2O + O 2 Using an enzyme catalyst The catalytic decomposition of hydrogen peroxide provides a range of project opportunities of varying length and complexity. CHEMISTRY REVIEW accepts no responsibility if Project Page is used in any way as a set of instructions. It is not intended to be a set of instructions for practical work and does not include a list of safety precautions. NOTE: Project Page is designed to help you think about your investigation. This Project Page first appeared in the September 1995 issue of Chemistry Review, Volume 5, Number 1.


 0 kommentar(er)
0 kommentar(er)
